| Enterprise Solutions | Instrument Services | Parts & Consumables | Support | Training | About Us | Contact Us |
USA phone for analytical instruments: 800-532-4752
USA phone for laboratory equipment: 1 888-860-5227, Option 3, then 1
Need contact info for other countries? Click here.

Laboratory Equipment Compliance Services
Open the door to sample integrity and confident compliance
Selecting the right compliance services solution is a significant part of your quality system. As you rely on quality results from our extensive range of equipment, including everything from centrifuges and pipettes to cold storage and water purification systems, you also can trust Unity Lab Services to be the compliance partner that helps you meet your regulatory assurance and documentation needs.
We provide compliance services for:
- Centrifuges
- Cold storage equipment
- Incubators
- Shakers
- Remote monitoring sensors
Our compliance and calibration services support organizations that rely heavily on equipment for critical sample storage and growth, and our protocols reflect a customized approach aimed at delivering robust and efficient regulatory compliance coupled with the desired degree of confidence.
Service benefits
- Industry-exclusive no-charge requalification guarantee: We are the only OEM to offer a guarantee and promise to re-qualify the unit at no charge if a key component fails while under the original product warranty or while equipment is under a qualifying service plan.* Coupled with our global reach and local support, Unity Lab Services is better positioned to offer consistent and comprehensive support compared to smaller, local, third-party service providers.
- OEM confidence: We’re trained in validation and equipment maintenance, so we fix audit issues others can’t.
- Proven efficiency: We help you pass qualifications the first time with our one-and-done best practices, which ultimately save resources and maintain operational uptime.
- Audit-ready calibration: Our services comply with ISO/IEC 17025 standards, giving you confidence that your calibration results will be both precise and accurate.
Lab equipment compliance services
- Installation Qualification (IQ): Documented verification that equipment is installed to manufacturers’ recommendations and/or user requirements.
- Operation Qualification (OQ): Documented verification that equipment (as installed) is operating as intended to manufacturer’s specifications.
- Performance Qualification (PQ): Documented verification that equipment can perform effectively and reproducibly based on approved specifications.
- Calibration: Periodic verification that equipment is producing accurate results within specified limits compared to traceable standards of measurement; best suited for cold storage equipment, CO2 incubators, centrifuges, water purification, and pipettes.
- Preventive Maintenance (PM): A standard set of procedures intended to ensure equipment performance is at or near the same levels achieved at the time of installation.
- Temperature Mapping (TM): Provides data measured at different temperatures and locations, door opening recovery tests, backup system testing, and a variety of checkpoints; best suited for cold storage and incubation equipment.
Recommended services for your regulatory needs:
| Application | Requirement | Recommended Services |
|---|---|---|
| Initial qualification for new equipment or change of service/status | I need my equipment qualified before I can use it | IQ/OQ/PQ bundle |
| Re-qualification | I need annual or periodic qualification | PM TM Calibration |
| Fleet programs |
I have multiple units and I need service done throughout the year | PM TM Calibration IQ/OQ OQ/PQ |
Turn to the industry leader
Contact us today to take advantage of our industry-exclusive no-charge requalification guarantee: If a key component fails while under the original product warranty or while equipment is under a qualifying service plan*, we will re-qualify the unit at no charge.
Coupled with our global reach and local support, Unity Lab Services is better positioned to offer consistent and comprehensive support compared to smaller, third-party service providers.
Recommended compliance services across the equipment life cycle
Unity Lab Services offers flexible compliance services that fit an organization’s unique needs. Regular maintenance plus frequent qualification, calibration, and preventive maintenance are recommended to lengthen equipment life and mitigate risks of non-compliance.
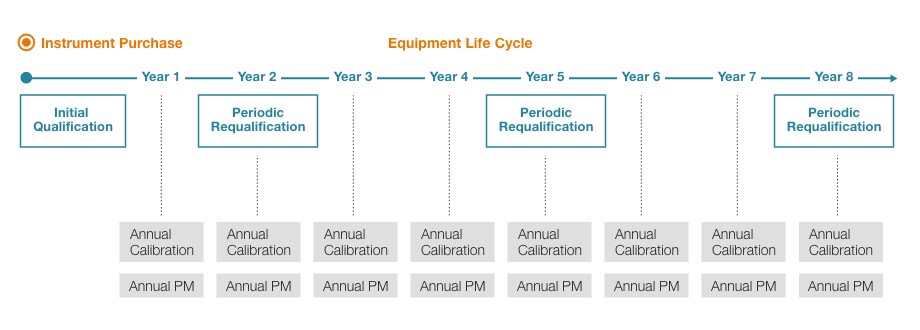
 |
Ensure your audit-readiness with our industry-exclusive, no-charge requalification guaranteeUnity Lab Services is the only original equipment manufacturer (OEM) service provider that offers a guarantee to re-qualify instruments and equipment at no charge if a key component fails while under the original factory warranty or a qualifying service plan.* Explore service plans |
*Terms and Conditions
Unity Lab Services Requalification Guarantee: Should your Thermo Scientific instrument or laboratory equipment require a repair while under a qualifying Unity Lab Services instrument service plan or warranty, we will requalify the repaired instrument or equipment at no additional cost. This guarantee does not cover optional repairs, relocations, consumables, or situations where a qualification test is interrupted due to customer intervention, power loss, or other situation out of the control of a factory-qualified field service engineer. Additional terms and conditions apply. All terms and conditions of service contract and factory warranty apply.
Additional Terms & Conditions:
Terms and conditions apply to Thermo Scientific analytical instruments, including mass spectrometry (LSMS, IOMS), chromatography (GC/GCMS, HPLC, IC/SP), trace elemental (ICPMS, ICPOES, AA), molecular spectroscopy, sample preparation, and discrete industrial analyzer (DIA) instruments where the operational qualification (OQ) has been "added on" to a support plan, basic, or extended warranty. Operational qualifications purchased as a billable service or under a factory warranty do not qualify for the no-cost requalification (RQ) and can be purchased à la carte at list price. Third-party instruments under Unity Lab Services contracts or engagements do not qualify for the no-charge requalification guarantee.
For Thermo Scientific laboratory equipment (centrifuges, cold storage equipment, incubators, shakers, and remote monitoring sensors) under a Total Care Service Support Plan, the requalification guarantee is applicable for the duration of the active contract period. For Thermo Scientific laboratory equipment under original product warranty, the no-charge requalification is valid for up to two years following the original qualification service event. Third-party instruments under Unity Lab Services contracts or engagements do not qualify for the no-charge requalification guarantee.